-40%
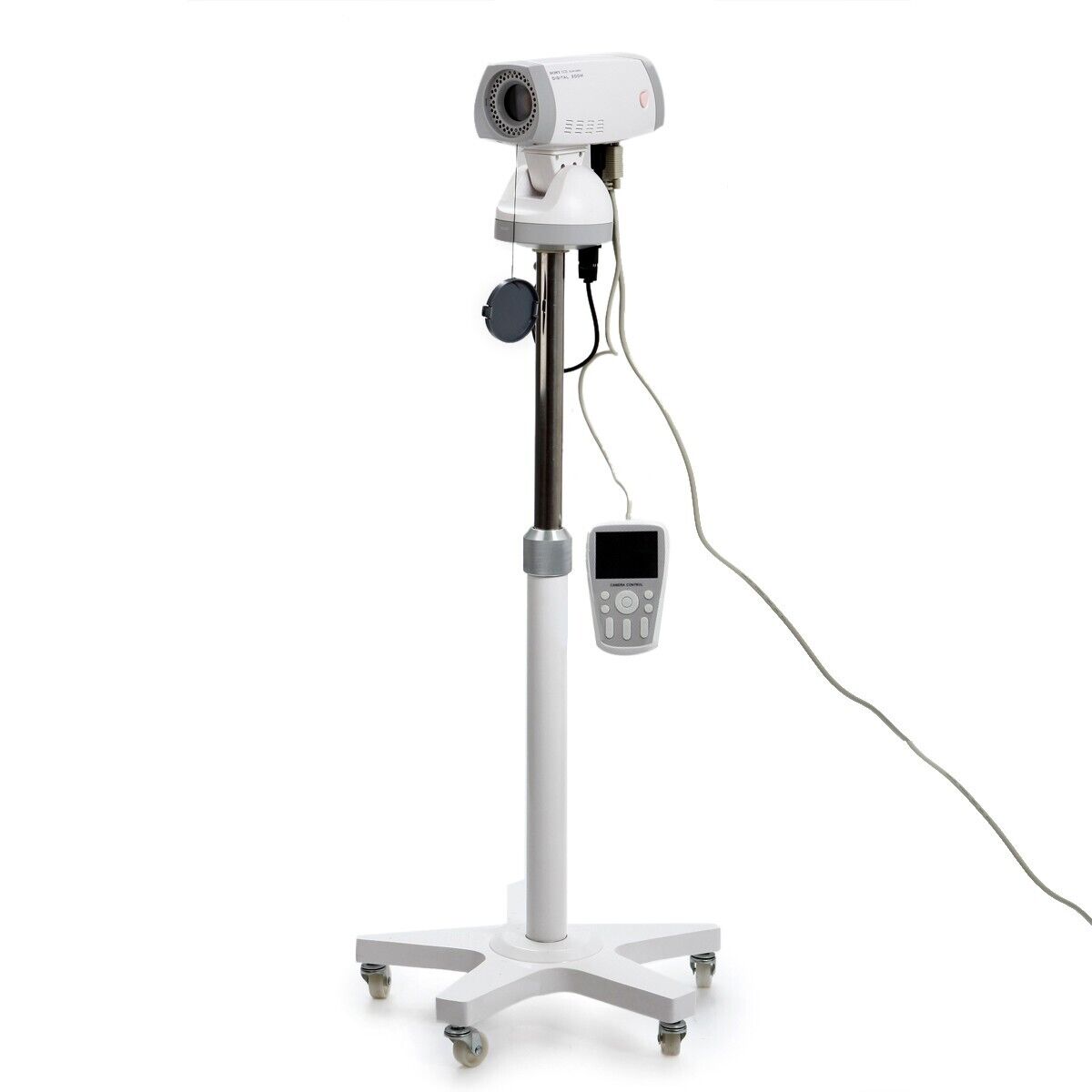
Electronic Video Colposcope Camera 850,000 Pixels +Tripod Medical CE
$ 611.95
- Description
- Size Guide
Description
Application:For clinical detection of pathological changes in vulva, vagina and cervis, filtering cervis cancer by fluorescence indirectly and images management.
RCS-500: - Camera 850,000 pixels
Work with Laptop & Desktop
Working Principle:
RCS-500 Digital Video Colposcope is applicable to detect pathological change in Vulva, vagina and the cervix, filtering cervix cancer by fluorescence indirectly, and images management.
Feature of Performance:
- The outlook is fashionable and convenient.
- We adopt the color digital CCD camera of high resolution and clarity. (-)
- The Colposcope is the sole dismountable light source, which has been got the patent.
- Bulbs adopt supper cold LED light source whose diameter is 3 mm. And in one light source there are 30 bulbs.
- There are freeze, filter, zoom in, zoom out, white balance button on the top of the camera for the speedy automatic function.
- Professional green-filter technique
- Image freeze
- White balance
- Auto focus
- Timing of acetic acid and iodine reaction tests
- Powerful image software and workstation system
Feature of Software:
- Capacity of freezing and storing static images continuously and a maximum of 99 images available for reviewing simultaneously in scroll.
- Capacity of cine loop and record continuously for different frequencies dynamic image in sampling.
- Capacity of comparison of 4 pictures in the screen, multi-orientation and dynamic consultation, copy, freeze, and other function.
- Supply hundreds of pathological reference image. Can help doctor compare the pathology and make the diagnoses.
- Need not be inputted because of abundant diagnosis information
- Capacity of internet report, and transmit the data through the internet to make an long-distance consultation and share of the information
- Color image report in multi-formats and freely input information to make the user template
- Tens of image enhanced functions to improve the checking rate of micro-focus
- Sole Filter function to make sure to see the vein clearly with less loss of the light.
- Easy operating software with icon, which can freeze and store 99 images consecutive and multi-orientation and dynamic consultation
Specification:
CCD
1/4 Super HAD CCD
Pixels
≥850,000 pixel
Illumination (min):
≤0.01Lux
Lens:
- 18 times focus
resolution ratio
1280*720
Light source
30-point ringed LED, lili-white, super bright, cool, parallel, shadowless, 100, 000 hours longevity
Focus
10mm
~
400mm
Zoom magnification
Optical ≥54X, Eelectronical ≥12X
Collection model
Electronic collection
Depth of field
5-200mm
Focalizing
Auto/Manual, quick
Signal-to-Noise
≥52 db
Color filter
Green
Image collection
Y
Cineloop
Y
Output port of the image
-Video/PAL/NTSC
Green Filter
Digital filter based on CCD imaging
Eliminate polarization
Y
Field of vision
Φ=2.5-320mm
Software package
Y
Operating system
Windows, VISTA
Standard configuration:
- Image capture system
- Image control system
- Bracket
- Two light source
- Software
- Plastic packing case
FDA Declaration:
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item unless you are an unauthorized purchaser. If the item is subject to FDA regulation, We will verify your status as an authorized purchaser of this item before shipping of the item. (The seller's name: Sara City: Beijing State: Beijing Country: China TEL.:86-18330168300).
This item has been cleaned and treated according to the manufacturer's instructions.
The Fingertip Pulse Oximeter is certified with the US FDA 510K No. K070371, the CE & TUV of Eureope and it is on the Australian Register of Therapeutic Goods (ARTG) with the code 136606.
The Powered Surgical Instrument / Speed 808 System is certified with the US FDA 510(k) Number:K132989
The Powered Surgical Instrument / Hair Remove Device is certified with the US FDA 510(k) Number:K180353
The Powered Surgical Instrument / Hair Remove System is certified with the US FDA 510(k) Number:K141973
massager, vacuum, light induced heating / Slimming Treatment Device is certified with the US FDA 510(k) Number:K161892